Updated on 5/4/2023. COVID-19 is an evolving topic and information may change. Most of the information comes from the Centers for Disease Control and Prevention (CDC) and the Public Health Communications Collaborative (unless otherwise noted).

Vaccine Information, Safety & Benefits
How were the COVID-19 vaccines created?
-
The technology around the mRNA (Pfizer and Moderna) vaccines has been in development for decades, but was brought to the market for the COVID-19 pandemic. The vaccine uses mRNA provides instructions to our immune system on how to recognize and fight against the COVID-19 virus should it come into contact with it. This does not alter your DNA. Learn more through this video by Johns Hopkins School of Public Health.
- The Johnson and Johnson vaccine uses an adenovirus technology. The virus has been altered so it can't make you sick, replicate or enter into your DNA. Learn more in this video from the Mayo Clinic.
-
Novavax vaccine is a protein-based vaccine, which is a vaccine technology that has been used for over 30 years. Other vaccines use the same technology, including Hepatitis B, HPV, tetanus, and influenza. This vaccine takes the spike protein and adds it to an immune boosting agent, called an adjuvant, to create an immune response in your body; and therefore, creates protective antibodies for your body to use if it comes into contact with the actual COVID-19 virus. To learn more about how the vaccine technology works, watch this brief overview. Or for a more in depth look at the vaccine, watch this video.
-
All COVID-19 vaccines have been rigorously tested and reviewed. The vaccine’s clinical trials three-phase process was detailed and thorough, and no shortcuts were taken. More than 150,000 people participated in U.S. clinical trials of the vaccines, and now, hundreds of millions of vaccine doses in the U.S. have been safely administered.
-
The Pfizer and Moderna vaccines have been granted full approval by the Food and Drug Administration (FDA). FDA approval requires a rigorous and structured review process, and it means that a vaccine or drug has cleared every level of review. On top of the rigorous testing and review that was required for emergency use authorization (EUA), for FDA approval the FDA requires even more data on safety, manufacturing, and effectiveness over longer periods of time and includes real-world data.
-
The Johnson & Johnson vaccine is still under the emergency use authorization by the FDA. Upon further review of a very small number of cases of a serious blood-clotting disorder connected to the Johnson & Johnson vaccine, both the CDC and FDA recommend the Moderna or Pfizer vaccines over the Johnson & Johnson unless a person is allergic to the mRNA vaccines or refuses the mRNA vaccines.
-
The Novavax vaccine is also only approved for emergency use authorization by the FDA. Clinical trial data show a small increased risk for myocarditis, an inflammation of the heart muscle. The risk of myocarditis from the vaccine is still less than the risk of myocarditis from COVID-19 infection. Find more information about the vaccine safety and ingredients here.
- As with all vaccines, there will be ongoing monitoring for adverse events among people who are vaccinated into the future.
- Learn more about the ingredients of each approved vaccine at the CDC's FAQ page.
Are the vaccines effective?
- All COVID-19 vaccines currently authorized for use in the United States helped protect people against COVID-19, including severe illness, in clinical trial settings. Studies that have looked at how COVID-19 vaccines work in real-world conditions (vaccine effectiveness studies) have shown that these vaccines are working well.
- People with moderately to severely compromised immune systems are especially vulnerable to COVID-19, and may not build the same level of immunity to 2-dose vaccine series compared to people who are not immunocompromised. Therefore, the CDC recommends that people with moderately to severely compromised immune systems receive an additional dose of mRNA COVID-19 vaccine at least 28 days after a second dose in order to improve immunocompromised people’s response to their initial vaccine series.
- Booster shots are now recommended for ages 12+ to help maintain strong immunity. Studies show that after getting vaccinated against COVID-19, protection against the virus may decrease over time and be less able to protect against the Delta variant. Although COVID-19 vaccination for adults aged 65 years and older remains effective in preventing severe disease, recent data suggest vaccination is less effective at preventing infection or milder illness with symptoms. Emerging evidence also shows that among healthcare and other frontline workers, vaccine effectiveness against COVID-19 infections is decreasing over time. This lower effectiveness is likely due to the combination of decreasing protection as time passes since getting vaccinated (e.g., waning immunity) as well as the greater infectiousness of the Delta and Omicron variants.
Who is eligible for the vaccine and how many doses are required?
- The Pfizer vaccine is approved for everyone 6 months and older. Three doses are administered for ages 6 months through 4 years. Two doses are administered for ages 5+.
- The Moderna vaccine is currently approved for ages 6 months-5 years, and ages 18+. All ages received 2 doses.
- The Johnson & Johnson and Novavax vaccines are currently approved for those over the age of 18. Johnson & Johnson is a one dose vaccine. Novavax requires two doses.
If I was already infected with COVID-19, do I still need the vaccine?
- Even if you have already had COVID-19, vaccination is an important step to protect yourself and those around you. One study showed that unvaccinated people who have had COVID-19 are still twice as likely as vaccinated people to get COVID-19 again.
What are the side effects of getting the vaccine?
-
Side effects are like other common vaccines. The most common side effects are pain/redness at the injection site, headache, fatigue, muscle/joint aches and low-grade fever.
-
Most side effects last less than 24 hours and those ages 55 and older reporter fewer side effects.
-
For more information, read the CDC guidance on side effects.
Can you get other vaccines at the same time or close to getting the COVID-19 vaccine?
-
Yes, you can get a COVID-19 vaccine and any other vaccines, including a flu vaccine, at the same visit. Experience with other vaccines has shown that the way our bodies develop protection, known as an immune response, after getting vaccinated and possible side effects of vaccines are generally the same when given alone or with other vaccines. Learn more about the timing of other vaccines.
Is the vaccine free?
- Yes, COVID-19 vaccines are available for everyone at no cost, including the Pfizer-BioNTech Vaccine for children ages 5 through 11 years. COVID-19 vaccines will continue to be given to all eligible people living in the United States, regardless of insurance or immigration status.
Can you still get sick with COVID if you are vaccinated?
-
Vaccine breakthrough cases are expected. COVID-19 vaccines are effective and are a critical tool to bring the pandemic under control; however, no vaccine is 100% effective at preventing illness. The risk of developing a severe case of COVID-19 or being hospitalized is significantly reduced for vaccinated individuals.
Can a person who is trying to get pregnant, is pregnant or breastfeeding receive the vaccine?
- Pregnant women are at a higher risk to get severely ill with COVID-19 compared with non-pregnant women, which can cause fetal complications. The CDC has issued a health alert recommending those who are pregnant, recently gave birth, are breast-feeding, or trying to become pregnant receive a COVID-19 vaccine as soon as possible.
- Data from thousands of people who are pregnant, breast-feeding and those who became pregnant after vaccination confirms that the COVID-19 vaccine is safe and effective before, during and after pregnancy. Data shows no increase in adverse outcomes in pregnant women who receive the COVID-19 vaccine. There is also no evidence linking vaccines to infertility.
- The risks of COVID-19 infection outweigh any known or potential risks of vaccination while pregnant or trying to conceive. See more information from the CDC and this shared decision making tool from UMass Medical School.
What should people who are immunocompromised do?
- Having a weakened immune system can make you more likely to get severely ill from COVID-19. Follow the CDC's guidelines for people who are moderately or severely immunocompromised.
- Take extra precautions from getting sick.
Do I still need to take precautions when I'm vaccinated and boosted?
- Yes, it's important to follow precautions, especially when transmission in your community is increasing.
- Monitor your Community Level to know the level of risk and recommended guidance.
- Get tested if you are symptomatic or exposed.
- Wear a high-filtration mask to reduce community spread, especially if you are symptomatic, were exposed, or are at high risk.
What should I do if I was exposed to someone with COVID or I test positive for COVID?
- It's important to isolate or quarantine, wear a mask, and monitor for symptoms. Learn more about what you can do here.
Booster Shots

Why are boosters needed?
-
Booster doses are common for many vaccines. COVID-19 vaccines continue to offer protection against hospitalizations and severe outcomes for people who are fully vaccinated. Protection against the virus may decrease over time and the efficacy of the vaccines is reduced against the rampant omicron variant.
-
As of September 2022, two newly available bivalent COVID-19 vaccines are recommened as a single-dose booster. A bivalent vaccine is designed to protect against two different strains of a virus. The new bivalent versions of the COVID-19 vaccine will offer protection against both the original strain of SARS-CoV-2 as well as the Omicron BA.4/BA.5 strain, which is now the predominant variant of the virus around the world.
Who is eligible for a booster shot?
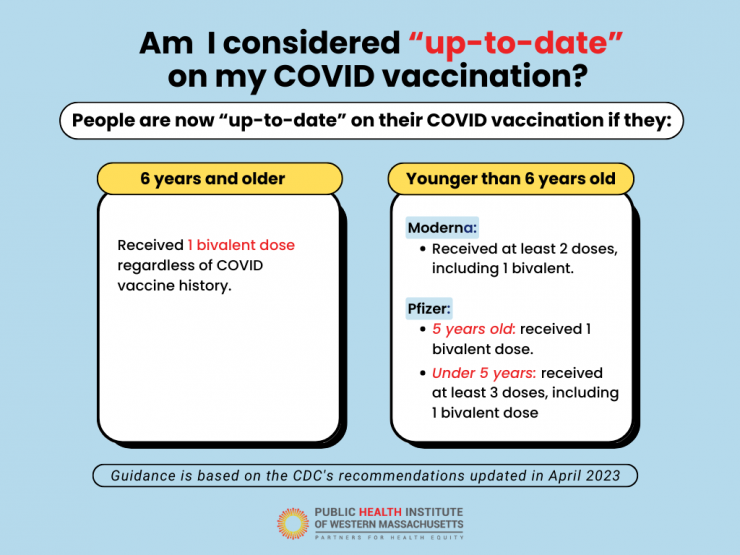
If you live in Massachusetts, anyone 5 years and older is eligible for a mRNA booster dose. See if you are up to date.
**For individuals who cannot or do not wish to get an updated/bivalent mRNA (Pfizer or Moderna) booster dose, they may opt to receive a monovalent Novavax booster dose if they: 1) are 18 years or older, and 2) have received their primary vaccine series but not any booster dose yet.
Can I mix and match my COVID-19 vaccine and booster?
-
Yes. Adults can mix and match between Pfizer and Moderna vaccines when getting boosted, as long as you meet age eligibility. For anyone who cannot or will not get a bivalent Pfizer or Moderna booster, they can choose to get a monovalent Novavax booster as long as you meet the Novavax eligiblity.
When and I considered “up to date” on my COVID vaccination?
-
As with vaccines for other diseases, protection is best when people stay up to date. Learn the latest guidance.
For more information on the COVID-19 booster shots, please refer to the following resources:
Vaccine for Children & Youth

Which children are eligible for the vaccine?
-
Children ages 6 months and older are eligible for COVID-19 vaccination through emergency use authorization (EUA).
-
Kids 6 months to 17 years old can choose between the Pfizer vaccine and the Moderna vaccine for this age group.
-
If your child has a special health needs or significant health issue – such as those resulting in a compromised immune system — check with their physician to determine if they should be vaccinated.
-
Learn more about the vaccines for children at mass.gov and the CDC.
How does the pediatric vaccine doses differ from the adult vaccines?
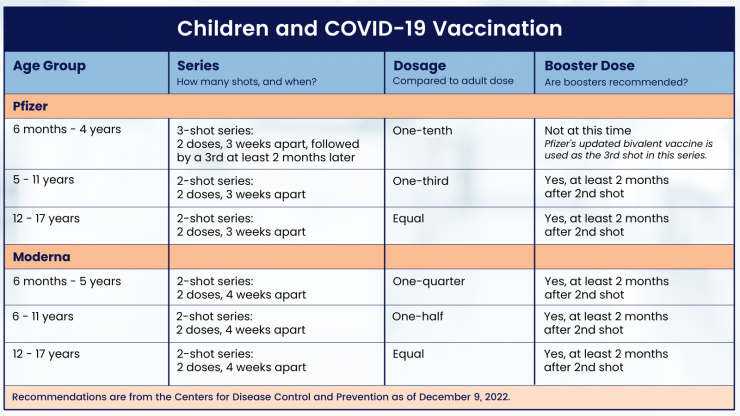
- The Moderna vaccine for children ages 6 months through 5 years old is a two-dose vaccine series, each one with 1/4 of the adult dose size. The second dose is given 4 weeks after the first dose.
- The Moderna vaccine for children ages 6 through 11 years old is a two-dose vaccine series, each one with 1/2 of the adult dose size. The second dose is given 4-8 weeks after the first dose.
- The Pfizer vaccine for children ages 6 months through 4 years old is a three-dose vaccine series, each 1/10 of the adult dose size. The second dose is given 3 weeks after the first, and the third dose is given at least 2 months after the second dose.
-
The Pfizer vaccine for children ages 5 through 11 years old is a two-dose vaccines series, each one with 1/3 of the adult dose size. The second dose is given about 3 weeks after the first dose.
-
The Pfizer and Moderna vaccines for youth ages 12 through 17 years old receive the same size dose as adult. The second dose of Pfizer is given about 3 weeks after the first dose. The second dose of Moderna is given 4-8 weeks after the first dose.
-
COVID-19 vaccine dosage does not vary by patient weight but by age on the day of vaccination.
Why should children be vaccinated?
- Children can still be infected with COVID-19, get severely sick from it, and spread it to others. COVID infection was one of the top 10 leading causes of death for kids in the U.S. Widespread vaccination is a critical tool to help stop the pandemic. Getting vaccinated will help children protect their family and community, as well as get back to the things they have missed: in-person school, playing with friends, and participating in sports activities.
Are the vaccines safe for children?
- Yes. The FDA and CDC have carefully reviewed the clinical trials with over 3,000 kids for the Pfizer’s COVID vaccine, and it has been proven to be safe and effective for children 12 and older. Serious side effects were rare. The vaccine goes through the same testing and clinical trials as all vaccines and no serious safety concerns have been identified. COVID-19 vaccines have undergone—and continue to undergo—the most intensive safety monitoring in U.S. history.

Are the vaccines for kids effective?
- Clinical trials show that the vaccines elicit a strong immune response after completing the recommended vaccine series. The vaccines are effective at reducing serious illness in adults and are expected to see the same result in children. The clinical trials show that the vaccines are less effective at preventing symptomatic infection, however, the virus has mutated since adult clinical trial sand the current subvariants have become more contagious. Booster shots may still be needed to remind the body’s immune system how to remain ready should it become infected.
- Learn more about the effectiveness of vaccines.
What are the side effects for children?
- Children may experience mild side effects, such as soreness in the arm, fatigue, headache, or a slight fever, and most will pass in one to two days. These side effects are normal signs that the body is building protection.
- Younger children reported less side effects than older children. No reports of myocarditis or pericarditis in kids under 5 years old.
- Serious side effects are rare and treatable. COVID-19 disease poses a higher risk of serious side effects than the vaccine.
- One side effect that parents and youth may feel concerned about it myocarditis or pericarditis (heart inflammation). These reactions are rare; in one study, the risk of myocarditis after the second dose of Pfizer-BioNTech in the week following vaccination was around 54 cases per million doses administered to males ages 12–17 years. Reported cases have occurred predominantly in male adolescents and young adults age 16 and older. Onset was typically within several days after mRNA COVID-19 vaccination, and cases have occurred more often after the second dose than the first dose. Patients who sought medical care for myocarditis/pericarditis responded well to medications and rest and had prompt improvement of symptoms. CDC and its partners continue to investigate these reports of myocarditis and pericarditis following mRNA COVID-19 vaccination to monitor safety.
- Parents/caregivers can enroll their child in v-safe, a free, easy-to-use smartphone-based tool that uses text messaging and web surveys to provide personalized health check-ins. Through v-safe, you can report how your child is feeling after vaccination.
- CDC recommends COVID-19 vaccination for everyone age 6 and older, given the risk of COVID-19 and related, possibly severe complications, such as long-term health problems, hospitalization, and even death. See the CDC's updated information on the clinical considerations on myocarditis and pericarditis after receipt of mRNA COVID-19 vaccines among adolescents and young adults.
Should a child get vaccinated even if they were already infected with COVID?
- Yes. The CDC reports that emerging evidence indicates that people can get added protection by getting vaccinated after having been infected with the virus that causes COVID-19. Even if a child has had COVID-19, they should still get vaccinated. For children who have been infected with COVID-19, their next dose can be delayed 3 months from when symptoms started or, if they did not have symptoms, when they received a positive test. This possible delay can happen with a primary dose or a booster dose.
What should a parent do if a child turns 12 after they get their first dose of the pediatric vaccine but before the second dose is due?
- As opposed to many medications, vaccine dosages are based on age and not size or weight. If a child turns from 11 to 12 years of age in between their first and second dose and receives the pediatric Pfizer-BioNTech COVID-19 Vaccine for their second dose, they do not need to repeat the dose.
Can the COVID vaccine impact child development?
- There is no clinical evidence to suggest COVID vaccines have effects on puberty or fertility.
Is it safe to co-administer COVID-19 vaccines with other vaccines, like flu?
- Yes, if a patient is eligible, both flu and COVID-19 vaccines can be administered at the same visit, as recommended by CDC and ACIP. In addition to flu vaccine, COVID-19 vaccine can be given with other vaccines as well.
Does the vaccine require parental consent?
- In Massachusetts, a legally authorized representative (usually a parent or guardian) must give permission (also called consent) for vaccination for under 18 years of age, such as by completing a written consent form that the minor can bring to their vaccination appointment. Please contact the vaccination location for more information on written consent, or download a copy of the consent form.
-
The parent or guardian does not need to go with the minor to their vaccination appointment to give consent. If the parent or guardian is not accompanying the minor, they should download and complete a pre-vaccination screening form, available at mass.gov/CDCScreeningForm. The form is available in several languages.
-
Parental consent laws may vary in other states.
What can parents do to help kids understand the pandemic and offer support?
- Below are some important reminders on how to support kids. Also, follow these great tips on how to talk with kids about the pandemic.
- Make kids feel safe. Stay calm and offer reassurance.
- Give them facts and let them lead the discussion. Don't overwhelm them with information they didn't ask for.
- Give them control and power where possible.
- Let them know what to expect.
- Empathize and allow them to express a range of emotions without judgment.
- Set your expectations and goals to match the child's developmental stage and temperament.
- Try to maintain a routine.
- Model behavior you want to see.
- Take care of yourself.

For more information regarding vaccines for children, go to:
- MA Department of Public Health vaccine info for ages 6 months - 5 years, ages 5-11 and ages 12-17
- Children & Youth with Special Health Needs
- Centers for Disease Control and Prevention
- Children and the COVID-19 Vaccine: The Conversation W. Kamul Bell talks with Pediatricians (video)


